Gilead Sciences, Inc.’s (NASDAQ:GILD) Kite and Kiniksa Pharmaceuticals, Ltd. (NASDAQ:KNSA) have entered a clinical collaboration to conduct a phase II, multicenter study on pipeline candidate mavrilimumab in combination with Yescarta.
We note that Gilead’s Yescarta was the first CAR T cell therapy to be approved by the FDA for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, and high-grade B-cell lymphoma and DLBCL arising from follicular lymphoma.
Mavrilimumab is an investigational, fully human monoclonal antibody that targets granulocyte macrophage colony-stimulating factor receptor alpha (GM-CSFRα). The combination study will be sponsored by Kite and conducted in patients with relapsed or refractory large B-cell lymphoma.
The objective of the study is to determine the effect of mavrilimumab on the safety of Yescarta.
Concurrently, Kite announced that it has submitted a Biologics License Application (BLA) to the FDA for the investigational CAR T cell therapy, KTE-X19, for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The BLA was based on data from the phase II ZUMA-2 study, which demonstrated an overall response rate of 93%, including 67% with complete response, as assessed by an Independent Radiologic Review Committee following a single infusion of KTE-X19.
We remind investors that KTE-X19 has been granted Breakthrough Therapy designation by the FDA and Priority Medicines (PRIME) by the European Medicines Agency (EMA) for relapsed or refractory MCL.
Kite plans to submit a Marketing Authorization Application for KTE-X19 in the European Union in early 2020.
A potential approval will boost Gilead’s portfolio even though the competition is stiff in the CAR T therapy space from the likes of Novartis’ (NYSE:NVS) Kymriah.
Gilead’s stock has gained 8.4% in the year so far compared with the industry's growth of 6.3%.
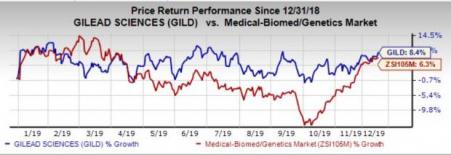
The massive decline in sales of the HCV franchise has propelled the company to focus on its HIV franchise, Yescarta and other newer avenues. The rapid adoption of Biktarvy maintains momentum in the HIV space amid stiff competition from the likes of GlaxoSmithKline (NYSE:GSK) . Gilead’s intent to foray into the inflammation market to diversify the revenue base is encouraging as well.
Gilead currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
Novartis AG (NVS): Free Stock Analysis Report
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Kiniksa Pharmaceuticals, Ltd. (KNSA): Free Stock Analysis Report
Original post
Zacks Investment Research
