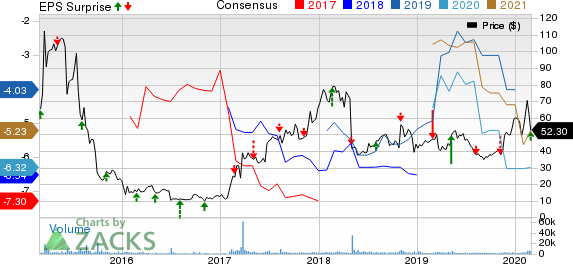
Esperion Therapeutics, Inc. (NASDAQ:ESPR) incurred a loss of $2.26 per share in the fourth quarter of 2019, narrower than the Zacks Consensus Estimate of $2.71. The company had incurred loss of $2.24 per share in the year-ago period.
The company generated revenues of $0.98 million, slightly ahead of the Zacks Consensus Estimate of $0.97 million. The company did not record any revenues in the year-ago quarter. Revenues in the reported quarter were mainly attributable to initial recognition of the upfront payment related to the commercial agreement with Daiichi Sankyo Europe, which Esperion signed in January 2019. The agreement granted exclusive rights to Daiichi Sankyo to commercialize Esperion’s bempedoic acid tablet, and a combination tablet of bempedoic acid and Zetia (ezetimibe) in Europe and Switzerland, following their potential approval from European Commission. Zetia is marketed by Merck (NYSE:MRK) as cholesterol lowering drug.
Earlier this month, Esperion received approval from the FDA for bempedoic acid tablet and bempedoic acid/Zetia combination tablet as a treatment for elevated LDL-C (bad cholesterol) in hypercholesterolemia patients. The single and combination tablets will be available under the brand names of Nexletol and Nexlizet, respectively.
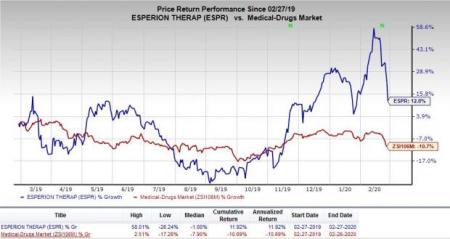
Despite encouraging quarterly results, Esperion’s shares were down 8.3% on Feb 27, following the earnings release presumably due to a significant decline in broader S&P 500 Index. However, the company’s stock has increased 12% in the past year against the industry’s 10.7% decrease.
Quarter in Details
Research and development (R&D) expenses decreased 22.8% from the year-ago period to $38.2 million.
General and administrative expenses were up 93.8% year over year to $21.7 million, primarily due to costs to support pre-commercialization activities for Nexletol and Nexlizet.
As of Dec 31, 2019, Esperion had cash, cash equivalents and investment securities of $201.7 million compared with $244.8 million as of Sep 30, 2019.
2019 Results
Esperion recorded revenues of $148.4 million in 2019. With no approved products and active collaborations, revenues were nil in 2018. Loss for the period was $3.59 per share, narrower than the year-ago loss of $7.54 per share.
2020 Guidance
Esperion provided its 2020 guidance for collaboration revenues and operating expenses. The company expects a milestone payment of $150 million from Daiichi Sankyo Europe. The company expects additional cash funding of $25 million under its funding agreement with Oberland Capital. The Zacks Consensus Estimate for 2020 revenues was $194.26 million.
The company anticipates R&D expense for 2020 to be in the range of $145-$155 million. Selling, general and administrative (SG&A) expense is expected to increase significantly year over year in 2020 to the range of $225-$235 million. These expenses exclude stock-based compensation. Increase in SG&A expenses was attributable to commercialization costs of its recently approved drugs.
However, the company did not provide any guidance for sales of its products – Nexletol and Nexlizet – in United Sales and royalties from their sales in Europe in 2020.
The company anticipates its current funds along with expected income and any product sales to be sufficient to fund operations.
Pipeline Update
Following the FDA’s approval for Nexletol and Nexlizet, the company plans to launch Nexletol next month and Nexlizet in July.
Last month, the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended approval of bempedoic acid tablet and bempedoic acid/Zetia combination tablet for a similar indication approved by the FDA. Decisions related to their approval from the European Commission is expected in the second quarter.
Esperion is planning to enter into a new development and commercial collaboration agreement for its hypercholesterolemia drugs in April.
Currently, the company is evaluating Nexletol and Nexlizet in the CLEAR cardiovascular outcomes study. The study is evaluating bempedoic acid for occurrence of major cardiovascular events in statin averse patients with or at high-risk of cardiovascular disease.
Our Take
Esperion reported encouraging fourth-quarter results. The company’s prospects received a boost with FDA approval for its two pipeline drugs, Nexletol and Nexlizet. European approval is expected next quarter. The U.S. launch of the drugs over the coming few months will likely create a steady revenue stream in the latter part of 2020. However, significant increase in operating expenses will hit the bottom line.
We note that clinical data and estimated list prices for Nexletol and Nexlizet seem competitive compared to other LDL-C lowering drugs including Amgen’s (NASDAQ:AMGN) Repatha and Sanofi’s (NASDAQ:SNY) Praluent. However, we remain on the sideline till data on launch uptake of the drugs are available.
Zacks Rank
Esperion currently carries a Zacks Rank #3 (Hold).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Sanofi (PA:SASY) (SNY): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Esperion Therapeutics, Inc. (ESPR): Free Stock Analysis Report
Original post