Duchenne muscular dystrophy (“DMD”) is a rare genetic disease and a severe form of muscular dystrophy, primarily affecting males. It progressively weakens and degenerates skeletal and heart muscles.
The symptoms of muscle weakness generally start to appear as early as four years of age in boys and progresses quickly.
Duchenne muscular dystrophy is one of the nine types of muscular dystrophy caused by the absence of a particular gene, dystrophin, which the body uses to keep the muscle cells intact. The disease starts to affect the muscles in the hips, pelvic area, thighs and shoulders in early stages and leads to degeneration of voluntary muscles in the arms, legs and trunk gradually. As the disease progresses, it affects the heart and respiratory muscles in early teen patients.
Life expectancy of DMD patients is as low as early adulthood. However, the scenario is changing with advances in cardiac and respiratory care with patients living till their 50s.
Due to the severity and rarity of DMD, the FDA is also incentivizing the companies developing treatments to treat this rare muscular degenerative disease by granting orphan drug designation to several candidates. This designation gives seven years of marketing exclusivity upon approval, research funding eligibility, tax credits for certain research costs, and a waiver of the FDA application user fee.
However, there are very few companies developing DMD treatments due to lower number of patients. Sarepta Therapeutics, Inc.’s (NASDAQ:SRPT) Exondys 51 is the first approved drug in the United States, which has been available since late 2016. The drug is a small molecule, which targets mutation of the DMD gene susceptible to exon 51 skipping. Exondys 51 and PTC Therapeutics, Inc.’s (NASDAQ:PTCT) Emflaza are among the approved treatments of DMD in the United States.
The focus is currently shifting to development of gene therapies for the treatment of the disease. Gene therapies deliver a functional copy of the dystrophin to muscle cells to restore its production.
3 Pharma/Biotech Companies Developing Gene Therapy to Treat DMD
Here we discuss three companies, which are researching and developing advance treatments for DMD.
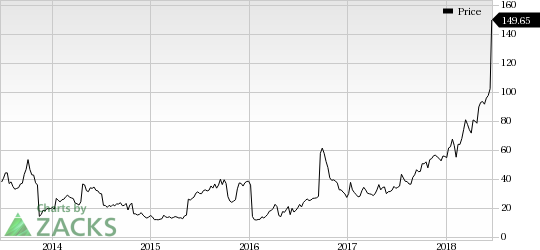
Sarepta has about eight exon-skipping candidates in its pipeline. Its lead candidate, golodirsen, is an exon-53 skipping candidate, which has demonstrated better efficacy than Exondys 51 with 100% response rate in a mid-stage study. A new drug application (“NDA”) is expected to be filed by the end of this year.
Apart from exon-skipping candidates, Sarepta is focusing on gene therapies for developing treatments for DMD. The company also initiated two phase I/II studies in the fourth quarter of 2017, which will evaluate gene therapies for DMD. Earlier this week, it announced promising preliminary results from an early-stage study evaluating the gene therapy, AAVrh74.MHCK7.micro-dystrophin. (Read more: Sarepta's Stock Soars on Encouraging Gene Therapy Results)
Sarepta currently carries a Zacks Rank #4 (Sell).
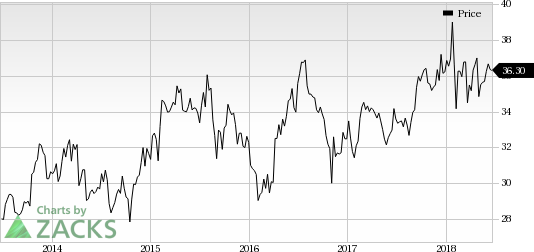
Pfizer Inc. (NYSE:PFE) is a large pharma company, which is also developing its gene therapy for the treatment of DMD. In April, the company initiated dosing of patients in a phase Ib study, which is evaluating its mini-dystrophin gene therapy candidate, PF-06939926, for treating DMD. The company is developing the therapy for the treatment of boys aged five to 12 years. Pfizer added the candidate to its pipeline with the 2016 acquisition of Bamboo Therapeutics. (Read more: Pfizer Starts Phase Ib for Duchenne Muscular Dystrophy Drug)
Pfizer currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
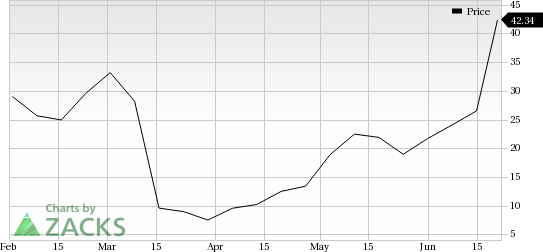
Solid Biosciences Inc.’s (NASDAQ:SLDB) lead candidate, SGT-001, is a microdystrophin gene transfer, which is being evaluated for the treatment of DMD. The candidate is in early stage of development. However, the company had received a setback with the FDA putting its phase I/II IGNITE DMD study – initiated during the fourth quarter of 2017 – under clinical hold in February. Earlier this week, the FDA lifted the clinical hold on this study, which boosted the company’s stock. (Read more: Solid Surges as FDA Lifts Clinical Hold on DMD Drug)
Solid Biosciences currently carries a Zacks Rank #4.
Apart from the above mentioned companies, Catabasis Pharmaceuticals, Inc. (NASDAQ:CATB) is developing its oral small molecule candidate, edasalonexent, for the treatment of DMD irrespective of the underlying dystrophin mutation.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana.
Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look.
See the pot trades we're targeting>>
Pfizer Inc. (PFE): Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT): Free Stock Analysis Report
Catabasis Pharmaceuticals, Inc. (CATB): Free Stock Analysis Report
PTC Therapeutics, Inc. (PTCT): Free Stock Analysis Report
Solid Biosciences Inc. (SLDB): Free Stock Analysis Report
Original post
Zacks Investment Research