The FDA Oncology Drugs Advisory Committee Meeting held today (May 2nd, 2013) to review the NDA submitted by Delcath Systems (DCTH) resulting in 16-0 vote against the Melblez Kit in terms of demonstrated benefits versus potential risks given the clinical trial materials included in briefing materials released on April 30th 2013.
The Melblez Kit, which was formerly known as CHEMOSAT, is a hybrid drug-device product that utilizes percutaneous hepatic perfusion (PHP) to deliver high doses of a chemotherapeutic agent directly to the liver. The product is designed to reduce the unwanted side effects of chemotherapy as a result of introduction of toxic agents into the rest of patients’ bodies while maximizing the efficiency of a chemotherapeutic regimen to patients’ livers. Specifically, the company chose Mephalan (aka Alkeran) as its chemotherapeutic agent.
From the briefing documents:
“Melphalan was selected as the chemotherapeutic agent for PHP treatment because it binds melanin precursors, is an alkylating agent with a steep dose response [Teicher et al, 1988], and has been used successfully in an analogous regional procedure, IHP, for treating unresectable hepatic metastases from melanoma”
Trading was halted just before 1:00 PM EST for shares of DCTH. As initially mentioned on the update on Catalyst~Watch update on April 30th, the market had already reduced the valuation of Delcath significantly after the release of the FDA briefing documents. DCTH is already down ~47% since Monday’s open, although the extremely negative vote on the Melblez NDA is likely to bring the stock even lower.
The PDUFA goal date for Melblez isn’t until September 13th 2013. While the FDA is not required to follow the advice of the advisory panel, it is extremely likely that Delcath will receive a CRL based on the adverse events that were seen during the clinical development process. The proposed REMS that was submitted along with the Melblez NDA was also deemed insufficient to mitigate the risks of a commercial launch of the Melblez Kit.
These risks are outlined with results from Phase I studies:
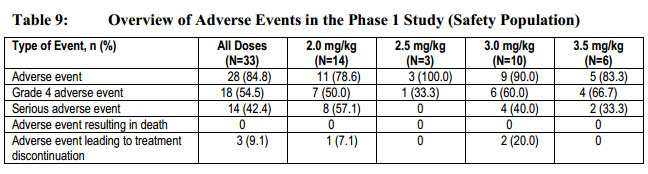
“The most common adverse events were hematologic events, including thrombocytopenia, neutropenia, febrile neutropenia, and anemia, and increases in liver function tests (bilirubin, AST, and ALT) (Table 26). Thrombocytopenia, neutropenia, and febrile neutropenia were predominantly grade 4 events whereas anemia was predominantly a grade 3 event.”
Details on the REMS:
A REMS for the melphalan/PHP System has been issued by FDA to communicate important safety messages and safe use conditions for melphalan/PHP treatment to healthcare providers by the following:
• Informing healthcare providers of the risks of hepatic failure, gastric ulceration,
coagulation/bleeding diatheses, and procedural complications associated with the
melphalan/PHP system drug/device combination product
• Ensuring dispensing of the melphalan/PHP system only to specially certified hospitals
• Ensuring only appropriately trained and certified team members (interventional
radiologist, anesthesiologist, perfusionist, and medical or surgical oncologist)
participate in procedural aspects and administration of melphalan/PHP treatment
As of their latest 10-K filing, reporting the company’s balance sheet up to December 31st 2012, the company held $23.7M in cash and cash equivalents and had total stockholder’s equity of $20 M. If investors ultimately believe that the Meblez Kit developmental program cannot be salvaged after a CRL, the stock may move closer to an enterprise value of zero. DCTH is likely to open lower tomorrow.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Delcath’s Melblez Kit Rejected (Profusely) By FDA
Published 05/03/2013, 01:57 AM
Updated 07/09/2023, 06:31 AM
Delcath’s Melblez Kit Rejected (Profusely) By FDA
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.
