Key opinion leaders reinforce patch potential
DBV Technologies' (PARIS:DBV) investor event underlined the medical need for Viaskin Peanut, with key opinion leaders (KOLs) highlighting that peanut avoidance is not sufficient, with a number of patients experiencing accidental ingestion. In addition, the KOLs emphasised patient enthusiasm for Viaskin Peanut, evidenced by almost 100% compliance and high levels of conversion to the VIPES follow-on trial. Finally, Viaskin Peanut’s safety was reinforced, with a low 5% VIPES drop-out rate and three positive safety assessments.
Avoidance is not adequate
Professor Franklin Atkinson (John Hopkins Asthma & Allergy Center) highlighted that despite strict peanut avoidance, accidental exposure is a major concern, with 40% of food-related anaphylaxis in patients with a history of severe reactions. 50% of children experience accidental ingestion within five years and 75% within 10 years, and the majority of the c 100 food-induced deaths in the US are in patients with a known allergy. These data reinforce the medical need for Viaskin Peanut.
Safety is key
Viaskin Peanut exposes patients to tiny quantities of peanut, hence safety will be a key element for success. DBV provided an updated safety summary from VIPES, with none of the 15 SAEs related to Viaskin Peanut (11 were during the food challenge and two were on accidental peanut exposure). To date there have been 12 drop-outs (5%), lower than the expected 15% rate. Three DSMBs (Data and Safety Monitoring Board) have been completed, with no safety concerns.
Patient enthusiasm for Viaskin Peanut
Dr Gordon Sussman (University of Toronto) relayed his experience of the ongoing VIPES Phase II trial. He highlighted that patient enthusiasm for a peanut allergy treatment is high, evidenced by the ease of recruiting patients into the trial, the high “roll-over” rate into the optional two-year follow-on trial (OLFUS-VIPES), with 22 of 24 (92%) patients electing to continue, and the low drop-out rate. In addition, patient compliance of almost 100% was noted, reinforcing this point.
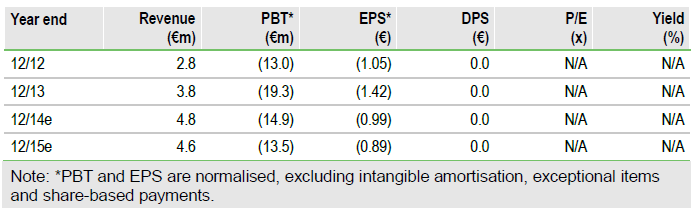
Valuation: Risk-adjusted NPV of €363m
Our underlying Viaskin Peanut assumptions are unchanged, with our rNPV now €363m (from €360m) owing to updated net cash and rolling our valuation forwards. Estimated net cash of €33.2m at end March should be sufficient to fund operations to end 2016.
To Read the Entire Report Please Click on the pdf File Below