Auris Medical Holding Ltd (NASDAQ:EARS) recently announced that the TRAVERS Phase II trial of AM-125 (intranasal betahistine) for treating acute vertigo is being initiated at study sites as regulatory and ethics committee approvals are coming in. An interim analysis of the data is expected in Q419. Also, its AM-201 Phase I trial for olanzapine-induced weight gain reached the midpoint in enrollment in early May and is on track for full enrollment by the end of June. Top-line data is expected in Q319.
Business description
Auris Medical is a Swiss biopharmaceutical company developing neurotology and central nervous system targeting therapeutics. It is developing intranasal betahistine in a Phase I trial for mental disorder supportive care and is entering Phase II for vertigo; both are designed to demonstrate proof-of-concept.
TRAVERS trial interim data in Q419
Auris is developing AM-125, an intranasal formulation of betahistine for the treatment of acute vertigo. As AM-125 bypasses the digestive tract where the oral compound is readily metabolized, the intranasal formulation has demonstrated superior bioavailability over oral betahistine. The Phase II trial, TRAVERS, will include 138 patients with surgically induced acute vertigo following vestibular schwannoma excision. The trial is currently being initiated at study sites with interim data expected in Q419.
AM-201 trial nearing full enrolment, data Q319
Auris also is developing AM-201, an intranasal betahistine formulation, for co-administration with olanzapine to counteract adverse effects such as weight gain and sleepiness. The company is currently enrolling the Phase I trial in Q119 in 50 healthy volunteers in Europe and is expected to complete enrolment this quarter. Data is expected in Q319.
A reverse split and capital raise
In May, the company conducted a 1-20 reverse split in order to regain Nasdaq listing compliance and also completed a public offering of shares and prepaid warrants with approximately $7.6m in net proceeds.
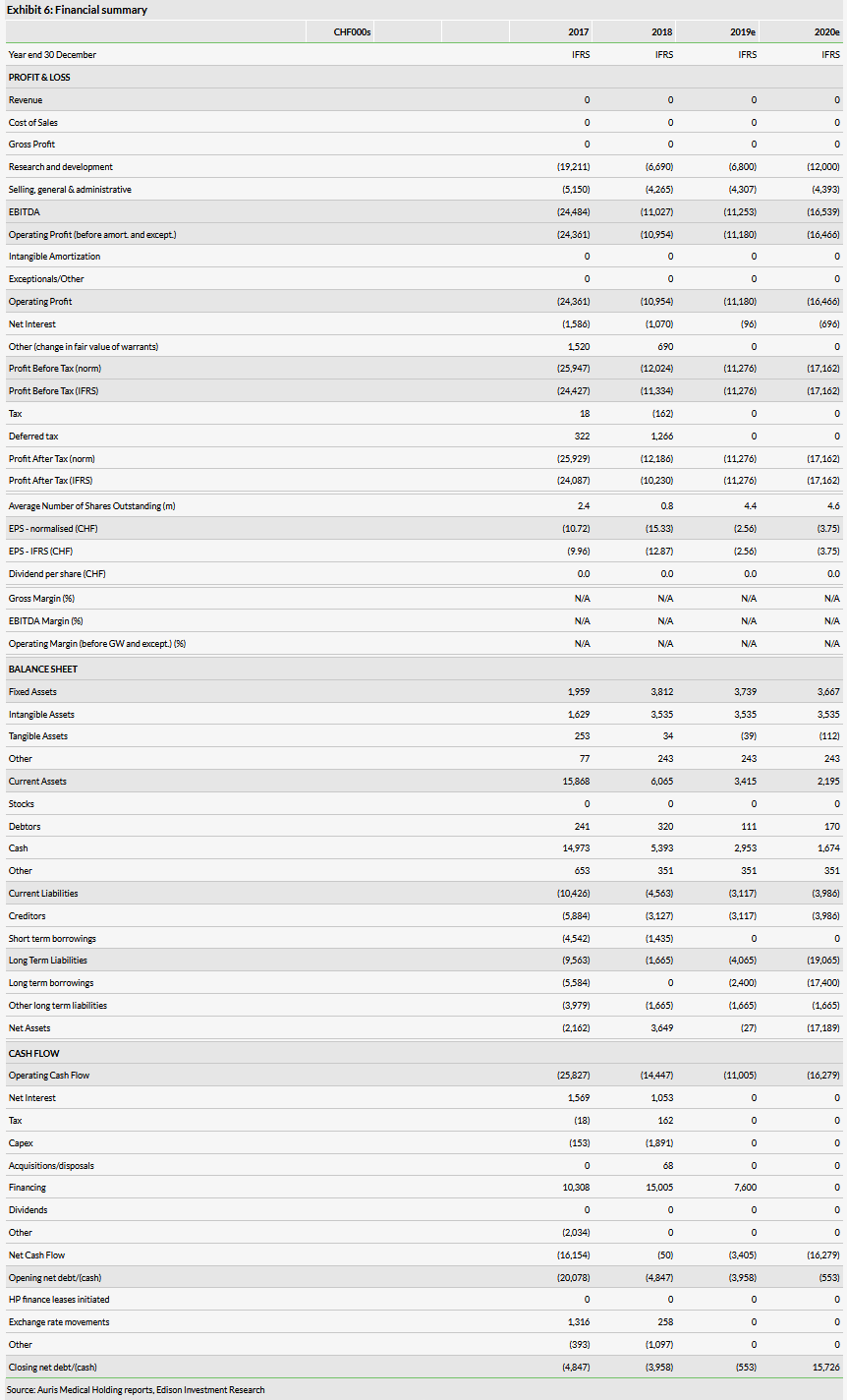
Valuation: $131.0m or $32.23 per basic share
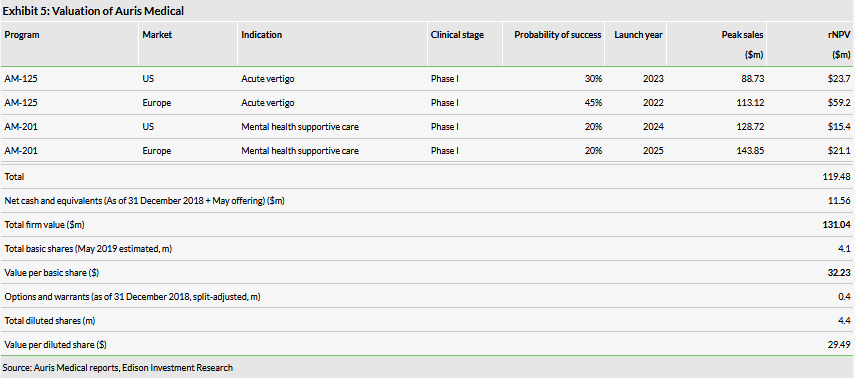
We have adjusted our valuation from $123.4m or $3.29 per basic share ($2.74 per diluted share), to $131.0m or $32.23 per basic share ($29.49 per diluted share). The increase in overall valuation was primarily due to a higher level of net cash while the increase in the per share value was due to the 1-20 reverse split, which was partially offset by the dilution from the equity raise.
AM-125 TRAVERS Phase II trial coming online
Auris recently provided an update on its AM-125 clinical program for the treatment of acute vertigo. The TRAVERS trial is a randomized, controlled, double-blind Phase II trial divided into two parts (Exhibit 1) and will include 138 patients in total with surgically induced acute vertigo following the removal of vestibular schwannoma (which is a noncancerous tumor on the main nerve leading from the inner ear to the brain, also known as acoustic neuroma). Vestibular schwannoma surgery leads to loss of peripheral vestibular input, which triggers acute vertigo.
In Part A of the trial, which the company is in the process of initiating, 50 patients will be administered AM-125 or placebo in five dose cohorts three times daily and 16 patients will receive 48mg oral betahistine three times daily (open-label, for reference purposes). Dosing will begin roughly three to four days after surgery. The company plans to report interim data in Q419 and expects to determine a dose-response curve and select a low dose and a high dose of AM-125 for the second part of the trial, which will be measured against placebo. Then in Part B of the trial, the company plans to enroll 72 patients.
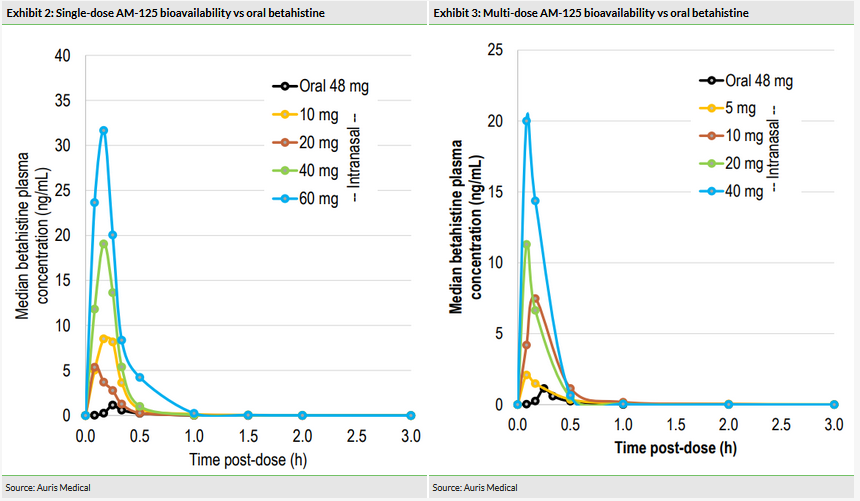
Auris previously demonstrated the superior bioavailability of AM-125, intranasal betahistine, compared to oral betahistine (48mg) in both single and multiple doses (Exhibits 2 and 3) in its Phase I trial. Adverse events (AEs) were mild to moderate, described as transient and included sneezing and nasal congestion, which corresponded to dose. One patient withdrew from the trial due to an AE, but no serious AEs were reported. According to Auris, the maximum tolerated repeated dose based on local tolerability in the nose was identified and set at 40mg; the maximum tolerated single dose was not reached at 60mg.
AM-201 for olanzapine-induced weight gain
Auris has also initiated the Phase Ib pharmacokinetics/pharmacodynamics (PK/PD) trial in AM-201, intranasal betahistine for the prevention of olanzapine-induced weight gain. Fifty healthy volunteers are currently being enrolled at one site in Europe and the trial was halfway through enrolment as of early-May. Enrollment is expected to complete by the end of June with data in Q319. The primary and secondary endpoints are weight gain and daytime sleepiness, respectively, whereas PK analysis will assess potential drug to drug interaction.
Valuation
We have adjusted our valuation from $123.4m or $3.29 per basic share ($2.74 per diluted share), to $131.0m or $32.23 per basic share ($29.49 per diluted share). The increase in overall valuation was primarily due to a higher level of net cash, while the increase in the per share value was due to the 1-20 reverse split, which was partially offset by the dilution from the equity raise. Note that the company has moved from quarterly to semi-annual financial reporting, hence we are using the 31 December 2018 cash level as our baseline.
Financials
As of 31 December 2018, Auris had CHF5.4m in cash and equivalents and CHF1.4m in debt. After the end of the quarter, Auris announced the full repayment of its loan facility with Hercules Capital, which eliminated the CHF1.4m in debt. In May, the company raised approximately $7.6m in net proceeds through the issuance of 440,000 shares of common stock and 1,721,280 in pre-funded warrants. In our forecasts, we model a total of CHF57.4m in financing needs through 2023 (previously CHF65m from which we subtracted the $7.6m in equity proceeds). We record this need as illustrative debt.