Cytori Therapeutics, Inc. (NASDAQ:CYTX) announced that two-year follow-up data from the SCLERADEC-I pilot study in patients with hand dysfunction associated with scleroderma when treated with single administration of Cytori Cell Therapy were published in the Current Research in Translational Medicine journal.
Data from the 12-patient, single-arm, open-label study showed that key clinical benefits were reported at the 6-month time point of the study were sustained at two years (follow-up range of 22–30 months). The primary endpoint of the study – Cochin Hand Function Score – improved 62.5% over baseline. The study also met the key secondary endpoints. In particular, from baseline, pain declined at two years, scleroderma-associated disability decreased 51.1% and Raynaud’s Condition Score improved 88.3%.
Additionally, key objective measures of scleroderma hand involvement demonstrated sustained benefit. While digital ulcers remained 60% lower than baseline, improvement in pinch strength was also sustained. Moreover, trends representing an improvement in hand mobility were reported. Meanwhile, it was noted that eight patients treated previously with prostanoids did not require subsequent re-treatment with these agents in the two-year follow-up period.
The long-term follow data is encouraging as it underscores the safety of single administration and benefits across multiple scleroderma symptoms.
Meanwhile, Cytori is evaluating the safety and efficacy of a single administration of Cytori Cell Therapy in a phase III study in scleroderma patients affecting the hands and fingers. The company anticipates 48-week follow-up data from this study in mid 2017.
The company is also evaluating Cytori Cell Therapy for additional indications including osteoarthritis of knee, stress urinary incontinence in men following surgical removal of the prostate gland and thermal burns combined with radiation injury.
CYTORI THERAPEU Price
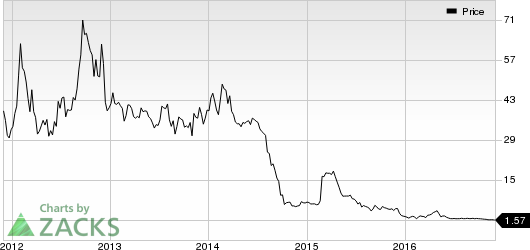
Meanwhile, a look at Cytori’s year-to-date share price movement shows the stock has dropped 43.4%, compared to the 23.5% decline for the Zacks categorized Medical/Biomedical Genetics industry.
Zacks Rank & Key Picks
Cytori currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the health care sector include Sucampo Pharmaceuticals, Inc. (NASDAQ:SCMP) , Vanda Pharmaceuticals, Inc. (NASDAQ:VNDA) and Anika Therapeutics Inc. (NASDAQ:ANIK) . While Sucampo and Vanda sport a Zacks Rank #1 (Strong Buy), Anika carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Sucampo’s earnings estimates increased from $1.03 to $1.22 for 2016 and from $1.30 to $1.58 for 2017 over the last 60 days. The company posted a positive surprise in all of the four trailing quarters with an average beat of 35.55%.
Vanda’s loss estimates narrowed from 68 cents to 52 cents for 2016, while its earnings estimates increased from 16 cents to 22 cents for 2017 over the last 60 days. The company posted a positive earnings surprise in three of the four trailing quarters with an average beat of 56.65%. Its share price has surged almost 81% year to date.
Anika’s earnings estimates increased from $1.96 to $2.06 for 2016 and from $2.03 to $2.09 for 2017 over the last 60 days. The company posted a positive surprise in all of the four trailing quarters with an average beat of 33.14%. Its share price has gained approximately 21% year to date.
Zacks' Top Investment Ideas for Long-Term Profit
How would you like to see our best recommendations to help you find today’s most promising long-term stocks? Starting now, you can look inside our portfolios featuring stocks under $10, income stocks, value investments and more. These picks, which have double and triple-digit profit potential, are rarely available to the public. But you can see them now. Click here >>
CYTORI THERAPEU (CYTX): Free Stock Analysis Report
ANIKA THERAPEUT (ANIK): Free Stock Analysis Report
VANDA PHARMACT (VNDA): Free Stock Analysis Report
SUCAMPO PHARMAC (SCMP): Free Stock Analysis Report
Original post
Zacks Investment Research