During volatile market conditions, many investors decide to sell to protect profit. Investors have seen extraordinary gains so far in 2013 and stocks that have done really well are seeing a small correction. This is healthy for the market and sets up another period for new buyers to come in. This week, we are detailing a company that we feel can still appreciate under these conditions because it is under speculation valued and under-the-radar.
Cempra (CEMP) is a biopharmaceutical company that is focused on developing antibiotics for the treatment of bacterial infectious diseases. Cempra's two main products in its pipeline are solithromycin (CEM-101) and Taksta. Solitromycin is in clinical studies for Community Acquired Bacterial Pneumonia (CABP) and urethritis. Taksta is in clinical studies for acute and chronic treatment of Methicillin-resistant Staphylococcus auereus (MRSA).
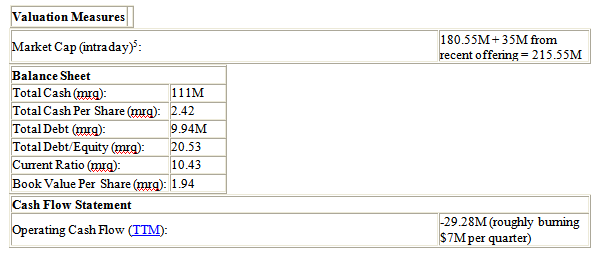
Solitromycin and Taksta are two products that have a large market potential. Both solitromycin and Taksta have oral and oral-suspension formulations. Cempra is the only company developing an oral formulation antibiotic for these indications. The company is also developing an oral suspension formulation that could have a broad use in pediatric care. With several catalysts later this year and a current market cap of about $215M, Cempra has lots of room for a run.
- Methicillin-resistant Staphylococcus aureus
- Taksta
Taksta, which was previously CEM-102, is being studied for the treatment of prosthetic joint injections (PJIs) caused by staphylococci, including MRSA. Taksta is a novel and proprietary dosing regimen of fusidic acid. The drug has been used for decades in Europe, Australia, and Canada, for joint infections, osteomyelitis, vertebral infections, septic arthritis, and other prosthetic and device related infections. The extensive use of the drug in other countries provides Cempra with about 40 years of established safety and efficacy profile data.
In 2007, there were about 200,000 hip replacements and 550,000 knee replacements. Cempra states that prosthetic joint infections occur in about 1% of patients with hip replacements and about 2% of patients who have undergone knee replacements, translating to an incident rate of about 10,000 a year in the US. The company gives a very detailed explanation of why Taksa will be an effective treatment:
· Taksta has demonstrated comparable efficacy to the only FDA-approved oral treatment for MRSA. In a Phase 2 trial in 155 ABSSSI patients comparing Taksta to linezolid, Taksta successfully demonstrated efficacy comparable to linezolid and confirmed its effectiveness against S. aureus and MRSA.
· Taksta is an oral therapy for all types of S. aureus, including MRSA. The leading treatments for prosthetic joint infections and ABSSSI caused by MRSA are available only in IV formulations.
· Taksta has a lower incidence of resistance due to our proprietary loading dose regimen. Our in vitro studies have shown that the reason for resistance to fusidic acid that was reported to occur during oral treatment outside the U.S. is that it was not dosed optimally. In addition, published studies of resistance during oral treatment with fusidic acid outside the U.S. conclude that the frequency of resistance is related to the lack of initial potency, which has been addressed in the past by combination therapy. Our innovative loading dose regimen minimizes the development of resistance by increasing the amount of drug initially delivered to the bacteria.
· Taksta can be used in patient populations not well served by current treatments. We believe Taksta could also be used for patients that are anemic, as well as patients on serotonergic drugs such as SSRIs who could be treated with an oral antibiotic, but for whom linezolid may not be a viable or convenient treatment option due to side effects and/or increased monitoring requirements. We intend to develop a pediatric formulation of Taksta to address the need for a safe and effective oral treatment of staphylococci and streptococci in children.
Taksta could be a better treatment option for both doctors and patients because it is an oral formulation. Vancomycin is the current therapy for PJIs and it is delivered intravenously. Oral formulations are more convenient and easier to administer inside or outside of the hospital setting. The oral formulation also allows Cempra to pursue other indications using fusidic acid. Fusidic acid has been very effective in treating many other indications around the world. It has been successfully used against acne vulgaris, osteomyelitis, acute bacterial skin and skin structure infections.
If granted approval down the road, Cempra should receive a New Chemical Entity (NCE) for Taksta. That will grant the drug 5 years of market exclusivity, with the potential of 10 because of the GAIN Act. If they are also able to get Orphan Status, the product could have 12 years of exclusivity. The long period of exclusivity would allow Cempra to capitalize on the market with very little competition.
- Solithromycin
Azithromycin (Zithromax) by Pfizer (PFE) is one of the only macrolides approved in the United States. Zithromax totaled about $1.1B in sales last year and is one of the best selling antibiotics. Solithromycin is chemically similar to azithromycin, but is 8-16 times more potent. In the recent 10K the company details why solithromycin will be an effective treatment:
· Solithromycin has demonstrated a favorable safety and tolerability profile. Solithromycin has been tested in over 490 subjects in our Phase 1 and 2 clinical trials and has been shown to be safe and well tolerated to date.
· Solithromycin has demonstrated comparable efficacy to the current standard of care. In our Phase 2 trial in 132 CABP patients comparing the oral formulation of solithromycin to levofloxacin, solithromycin successfully demonstrated efficacy comparable to levofloxacin. In addition, solithromycin has been highly effective in a Phase 2 trial of bacterial urethritis.
· Solithromycin is potent against a broad range of bacteria and has excellent tissue distribution and intracellular activity. In pre-clinical studies, solithromycin was shown to be generally eight to 16 times more potent against respiratory tract bacteria in vitro than azithromycin as well as retains activity against azithromycin resistant strains. These pre-clinical studies also showed that solithromycin is potent against many bacteria that are resistant to levofloxacin and other fluoroquinolones.
· Solithromycin has a greater ability to fight resistant bacteria and to overcome resistance development due to its chemical structure. Solithromycin has a unique structure that binds to bacterial ribosomes in three sites while earlier generation macrolides have only one or two binding sites. Therefore, bacteria must mutate at three sites on the ribosome to become resistant to solithromycin. To date, we have seen no resistance to solithromycin in our clinical trials, and resistance was rare in our pre-clinical studies.
· Solithromycin has the potential for IV, oral and suspension formulations. We are developing oral and IV formulations to allow patients with severe CABP to be treated in both hospital and out-patient settings. Providing both the IV and oral formulations will enable IV-to-oral step-down therapy. We believe this would be more convenient and cost-effective for patients and provide pharmacoeconomic advantages to health care systems. We intend to develop a suspension formulation for treating bacterial infections in the pediatric population.
· Solithromycin has improved anti-inflammatory qualities. Our pre-clinical data suggest that solithromycin could have significantly greater anti-inflammatory properties than azithromycin and clarithromycin, which are used to treat patients with cystic fibrosis, or CF, and chronic obstructive pulmonary disease, or COPD, primarily for their anti-inflammatory properties.
Cempra is studying Solithromycin for community-acquired bacterial pneumonia (CABP) and plans to have pivotal phase III data by mid 2014. That data release should be a huge catalyst for the company's stock in the future. What is also interesting is that solithromycin has been used to treat patients with gonorrhea. Cemprus constructed a 22-patient trial and showed it was 100% effective in ridding patients of gonococcal infections. This study has peaked some major interest, since gonorrhea has become extremely difficult to treat throughout the world. Most strains of gonorrhea have become resistant to many of the drugs that are available. In Japan, scientists even discovered a strain that was resistant to all know forms of antibiotics.
More gonorrhea therapies are coming off the market due to drug resistance, with Suprax being the most recent. According to the Center of Disease Control, Roche's Ceftriaxone (Rocephin) is the only major treatment left. Rocephin has estimated peak sales of over $1 billion. Cempra has the chance to grab a chunk of this market if they can prove solithromycin is an effective treatment. Cempra has already spoken to the FDA about advancing solithromycin for gonococcal infections. In a recent press release the company stated:
A single Phase III study of solithromycin in uncomplicated gonococcal infection would likely be sufficient if the Phase 3 CABP trials are successful. This study would be planned to include 250 patients who receive solithromycin, of which 150 are males. Treatment of Chlamydia may be included as a secondary endpoint. The specific protocol remains under discussion.
Resistant strains of gonorrhea have become a severe global concern. Since there are not a plethora of effective drugs on the market, Cempra has an opportunity to develop a drug that is able combat the new strains. If the company were able to show positive data in a Phase III study for this indication, it would be huge news because a drug for this indication could generate large revenues.
- Catalysts and Milestones

The company is expecting to announce Phase II topline data of Taksta for prosthetic joint infections in the fourth quarter of 2013. The company has also announced positive news over the last couple months that pave the way for the company's future. On May 13, Cempra announced signing an exclusive license and development agreement for solithromycin with Tomaya Chemical Co., a subsidiary of FUJIFILM Holdings Corporation in Japan. They also announced the notification of patent allowance for the Taksta loading dose formulation, which will protect it until 2029. With lots of positive momentum going forward, it's only a matter of time before investors start to realize Cempra's potential.
- Financial Structure and Public Offering
Since Cempra's initial public offering (IPO) in 2012, the company has been quietly leveraging several governmental policies to its advantage. The Generating Antibiotic Incentives Now (GAIN) Act was just enacted by Congress in October 2012 to encourage and incentivize companies to create new and effective antibiotics. One very important benefit from this act includes an additional 5 years of market exclusivity for companies who develop treatments that qualify under the act. Also, Cempra just signed a 5-year contract with the Biomedical Advanced Research Development Authority (BARDA) for $58M. With the money generated from the public offering and $17M this year from the BARDA contract, Cempra has enough cash to run on for the next 3 years.
Many investors and traders tend to "knee-jerk" react negatively when offerings are announced by developmental biotechs. However, many of these offerings are very positive for both long and short term stock price appreciation. For example:
Trius Therapeutics (TSRX) did a public offering earlier this year that was received well by investors. The company is developing Tedizolid for the treatment of gram-positive infections, including MRSA. After the public offering, the stock ran from under $5 to over $9. We have written 2 articles on Trius, with our sentiment being that Trius provides an excellent speculation investment opportunity, and is an acquisition target moving forward.
On February 5, 2013, Celldex Therapeutics (CLDX) entered into an underwriting agreement Jefferies & Company, Inc. and Leerink Swann LLC, to sell 12,000,000 shares of its common stock at a price of $7.50 per share, subject to customary closing conditions. The offering closed on February 11, 2013. The offering went very well, with the company's stock price steadily appreciating since then, likely because Celldex is currently developing two very important unmet need drugs.
Rindopepimut, an immunotherapy that targets the tumor specific oncogene called EGFRvIII, and CDX-011, a fully-human monoclonal antibody-drug conjugate (ADC) that targets glycoprotein NMB (GPNMB). CDX-011 might have the most potential for Celldex, as it targets GPNMB. GPNMD is a protein overexpressed by multiple tumor types, including melanoma, breast cancer and gliomas, which have huge market potential -- Celldex currently sells for $14.06 a share.
On January 24, 2013, Ariad Pharmaceuticals (ARIA) entered into an underwriting agreement with J.P. Morgan Securities LLC, Cowen and Company, LLC and Jefferies & Company, Inc. to sell 15,307,000 shares of the Company's common stock at a public offering price of $19.60 per share. The offering closed on January 29, 2013, with Ariad receiving net proceeds to the Company of approximately $287.5 million. Since this most recent offering, the company's stock reached a high of $22.49 on March 14, 2013.
Ariad is currently focusing on commercializing Iclusig (formely known as ponatinib), which is a cancer medicine; and is also developing AP26113, a small molecule that in preclinical studies has exhibited activity as a potent tyrosine-kinase inhibitor of anaplastic lymphoma kinase (ALK), epidermal growth factor receptor (EGFR), and c-ros oncogene 1 (ROS1).
Ariad also engaged in am successful offering in December of 2011, raising over $200M at the time at $12.50, which within a year, the stock doubled to over $25.
As we can clearly see with the 2 examples we have given, when a developmental biotech is working on important drugs, investors will in fact support offerings, and not necessarily sell into it. Cempra is very much "under the radar," and we feel that the company presents a very good opportunity for both traders and biotech speculation investors.
- Management and Insider Ownership

Insiders hold a nice position in Cempera, which is always a bullish sign to see. Dr. Goldstein in particular holds a massive 3 million + share position.
When engaging in secondary offerings to raise cash, companies with higher insider ownership tend to work harder and smarter to secure financing that is beneficial to themselves as shareholders, which is a positive for both retail and institutional shareholders. Far too many developmental biotechs do not have strong insider ownership, and often times these companies tend to needlessly over dilute, which is not beneficial for shareholders.
Conclusion
Cempra's secondary offering has gone well so far, receiving solid support. The company now has enough cash on hand to fund operations for the next 3 years. It's our opinion that Cubist (CBST) is likely to come knocking on Cempra's door soon to inquire about a partnership and/or to acquire the company. Cubist made an unsolicited offer earlier this year for another company in the same segment. Trius, as we mentioned in a prior article, should also peak Cubist's interest for partnership and/or acquisition. Cubist certainly seems more than willing to acquire a smaller company within its segment, and Cempra and Trius are the likely targets in our mind.
Since Cempra has high insider ownership, with such insiders as Dr. Goldstein holding huge positions, we feel the company will work hard to either get a solid partnership, or shop the company around for acquisition.
Our 1 year target price for Cempra based on its current $215M market cap is over $500M, or basically around $15 a share. Based on longer term potential, we feel an acquisition price around $1B is warranted, which would roughly equate to $30 a share. All of this is dependent on good Phase II and Phase III results, which will be announced in the fourth quarter of 2013 and early 2014, respectively.
Disclosure: I am long CEMP. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it. I have no business relationship with any company whose stock is mentioned in this article.
Additional disclosure: Disclaimer: This article is intended for informational and entertainment use only, and should not be construed as professional investment advice. They are my opinions only. Trading stocks is risky -- always be sure to know and understand your risk tolerance. You can incur substantial financial losses in any trade or investment. Always do your own due diligence before buying and selling any stock, and/or consult with a licensed financial adviser.
