Bristol-Myers Squibb Company’s (NYSE:BMY) first-quarter 2018 earnings of 94 cents per share exceeded the Zacks Consensus Estimate of 85 cents and the year-ago quarter earnings of 84 cents.
Total revenues of $5.19 billion were slightly higher than the Zacks Consensus Estimate of $5.18 billion and increased 5% from $4.93 billion recorded in the year-ago period. Strong sales of Opdivo and Eliquis contributed to the top line in the reported quarter.
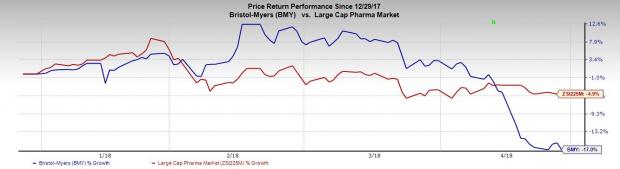
Shares of the company were up almost 0.5% in pre-market trading, possibly on better-than expected earnings. However, Bristol-Myers’ shares have declined 17% so far this year, which compares unfavorably with the industry’s decrease of 4.9%.
Quarterly Details
Revenues were up 1% year over year when adjusted for foreign exchange impact. Revenues increased 1% to $2.8 billion in the United States and 10% outside the country. Ex-U.S. revenues were up 1% when adjusted for foreign exchange impact.
Leukemia drug Sprycel raked in sales of $438 million, down 5% year over year. U.S. sales for the drug were down 13% to $214 million. A label expansion of the drug in pediatric patients in the United States last November presumably did not have much impact on sales.
Rheumatoid arthritis drug, Orencia, was up 11% in first-quarter 2018 to $593 million. Melanoma drug, Yervoy contributed $249 million to the top line during the reported quarter, down 25%. U.S. sales for the drug were down 33% to $162 million.
Opdivo, which is approved for multiple cancer indications, generated revenues of $1.51 billion, up 34% from the year-ago period.
Sales of cardiovascular drug, Eliquis, were $1.51 billion during the reported quarter, up 37% year over year. Multiple myeloma drug, Empliciti recorded sales of $55 million, up 4% year over year.
However, the performance of key drugs in the Virology unit continues to disappoint. Sales of Baraclude declined 20% to $225 million. The Reyataz and Sustiva franchises deteriorated 36% and 54% year over year to $124 million and $84 million, respectively. The Hepatitis franchise lost 98% of its year-ago quarter sales and contributed a mere $3 million to revenues.
Research and development (R&D) expenses in the quarter decreased 4.1% to $1.25 billion. Marketing, selling and administrative expenses declined 9.7% to $980 million.
Gross margin was 69.5% in the quarter compared with 74.3% in the year-ago quarter due to change in product mix.
Regulatory Update
Subsequent to the quarter in April, the FDA approved Opdivo+Yervoy combination regimen for treating intermediate- and poor-risk advanced renal cell carcinoma in patients who have not received any prior treatment. In March, the FDA had granted priority review to a supplemental biologics license application (sBLA) seeking approval of the combination regimen for treating microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer.
Also, in April, the FDA granted priority review to the sBLA seeking approval of Opdivo monotherapy in patients with small cell lung cancer in third or later line setting. A new dosing regimen for Opdivo, 480 mg every four weeks, was also approved for the majority of indications.
Collaborations
In April 2018, Bristol-Myers collaborated with Johnson & Johnson’s (NYSE:JNJ) subsidiary, Janssen, to develop and commercialize its Factor Xia inhibitor program, including BMS-986177, for treating major thrombotic conditions. In the same month, Bristol-Myers and privately-held Illumina (NASDAQ:ILMN), Inc. announced a collaboration to utilize the latter’s next-generation sequencing technology.
In February, Bristol-Myers Squibb and Nektar Therapeutics (NASDAQ:NKTR) entered into a strategic development and commercialization collaboration for Nektar’s lead immuno-oncology program, NKTR-214. Per the collaboration, the companies will develop and commercialize NKTR-214 in combination with Opdivo and Opdivo plus Yervoy in more than 20 indications across nine tumor types, as well as potential combinations with other anti-cancer agents from either of the respective companies and/or third parties. Both the companies plan to initiate studies in renal cell carcinoma and melanoma in mid-2018.
Pipeline Update
In April 2018, Bristol Myers announced data from a phase III study, Checkmate-227, which showed superior progression-free survival in first-line non-small cell lung cancer (“NSCLC”) treated with Opdivo – Yervoy combination compared to chemotherapy. In January 2018, Bristol-Myers announced positive data from Phase II CheckMate-142 study evaluating the combination of Opdivo and Yervoy in patients with DNA mismatch repair deficient (dMMR) or microsatellite instability-high (MSI-H) metastatic colorectal cancer (mCRC).
The company also announced data from the phase III study, CheckMate-078, evaluating Opdivo in Chinese population. Data showed that the drug achieved superior overall survival in previously-treated patients with NSCLC as compared to docetaxel.
Data from a long-term follow up phase III study – CheckMate -141 – showed that Opdivo reduced risk of death by 32% in metastatic squamous cell carcinoma of the head and neck after a minimum two years of follow-up compared to standard chemotherapy.
In March, Bristol-Myers along with Pfizer Inc. (NYSE:PFE) announced data from a real-world data analysis of different direct oral anticoagulants including Eliquis, J&J’s Xarelto and Boehringer Ingelheim’s Pradaxa. Data showed that Eliquis achieved significantly lower rates of both stroke/systemic embolism and major bleeding compared to both Xarelto and Pradaxa.
2018 Guidance Updated
Bristol-Myers increased its adjusted earnings expectations for 2018. The company now projects earnings in the range of $3.35 to $3.45 per share (previously $3.15 to $3.30). The Zacks Consensus Estimate for earnings is pegged at $3.22. The company expects worldwide revenues to increase in mid-single digits.
Our Take
Bristol-Myers beat earnings expectations, primarily on robust sales of Opdivo and Eliquis in the quarter. The increase in 2018 guidance for adjusted earnings was also encouraging. We are positive on Bristol-Myers’ efforts to develop its pipeline, especially Opdivo. Several label expansion applications for Opdivo are under review in the United States and Europe. Potential approval will further boost the prospects of this blockbuster drug. Also, superiority of Eliquis in real world data analysis is expected to further boost sales of the drug.
Bristol-Myers carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Nektar Therapeutics (NKTR): Free Stock Analysis Report
Original post