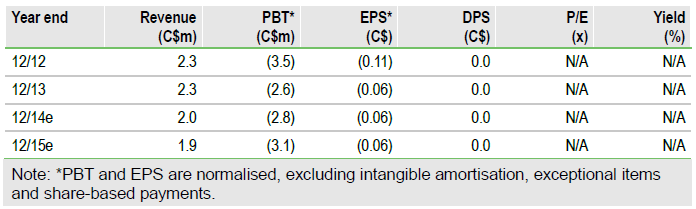
Moving forward in rare diseases
BELLUS Health Inc.'s (TO:BLU) lead candidate, Kiacta, is in a pivotal Phase III trial for AA amyloidosis, an orphan drug indication affecting up to 25,000 patients worldwide. We estimate the probability of success at 60%, given positive efficacy trends in a previous Phase II/III study and modifications in the ongoing pivotal study to increase its statistical power and target more responsive patients. We determine an rNPV valuation of C$102m, compared to Bellus’ EV of about C$26m. The potential for premium pricing and a seven- to 10-year exclusivity period underscore the investment case. Bellus is fully funded beyond the Kiacta study results expected in 2016.
Pivotal study builds on earlier efficacy trends
In a Phase II/III study, Kiacta showed a 13% lower incidence of “worsening events” vs placebo after 24 months (p=0.06). Under a special protocol assessment with the FDA, Kiacta may obtain regulatory approval if it can show a statistically significant improvement in the time to persistent “worsening events” of kidney function vs placebo in the pivotal study. Given that this study will record nearly double the number of events (120) and that it pre-selects patients with proteinuria (who are more responsive to treatment), the drug should have a greater chance of success.
Auven partnership limits Bellus’s financial burden
Bellus entered a partnership in 2010 with Auven Therapeutics to fund the Phase III study, which started in December 2010 and will enrol approximately 230 patients. As of 26 April 2014, approximately 227 patients have been enrolled; Bellus expects recruitment to be completed in H114, with study completion in 2016. Bellus and Auven will share the proceeds approximately equally from any out-licensing deal(s) on positive completion of the study. We project a US market launch in H217, and that Bellus will be entitled to receive 12.5% of Kiacta sales on approval.
Valuation: rNPV of C$102m reflects upside
Our rNPV of C$102m applies a 12.5% cost of capital and assumes a 60% probability of success of Kiacta in AA amyloidosis and 10% in sarcoidosis (a secondary indication to start Phase II studies in Q414). After including the sarcoidosis opportunity and raising our treatment price forecasts to better reflect the pricing range for conditions with similar prevalence rates, our rNPV increases to $102m (up from $40m previously). Our per-share valuation is C$2.45 (basic) or C$1.85 (fully diluted), including C$14.1m net cash (at 31 March 2014).
To Read the Entire Report Please Click on the pdf File Below