AstraZeneca Plc. (NYSE:AZN) and partner Merck & Co., Inc. (NYSE:MRK) announced that the FDA has approved a label expansion of its advanced ovarian cancer drug Lynparza. The FDA has approved Lynparza as a first-line maintenance therapy for BRCA-mutated advanced ovarian cancer. It is the first regulatory approval for a PARP inhibitor in first-line maintenance setting.
We note that Lynparza is already marketed in the United States for platinum-sensitive relapsed ovarian cancer, regardless of BRCA status and germline BRCAm HER2-negative metastatic breast cancer.
The approval in the first-line setting was based on data from the pivotal phase III SOLO-1 study, which demonstrated that Lynparza (olaparib) tablets led to a statistically-significant and clinically-meaningful improvement in progression free survival (PFS) compared to placebo in women with BRCA-mutated (BRCAm) advanced ovarian cancer who were in complete or partial response following first-line standard platinum-based chemotherapy.
In the Lynparza arm, 60% of the patients were progression free at 36 months compared with 27% in the placebo arm. Lynparza reduced the risk of disease progression or death by 70% compared to placebo, following response to platinum-based chemotherapy.
With the approval for expanded use in the first-line setting, sales of Lynparza can improve in the future quarters.
Other PARP inhibitors available in the market are Tesaro, Inc.’s (NASDAQ:TSRO) Zejula and Clovis Oncology, Inc.’s (NASDAQ:CLVS) Rubraca. Zejula and Rubraca are also being evaluated in late-stage studies in the first-line maintenance setting for the treatment of ovarian cancer patients who have responded to platinum-based chemotherapy.
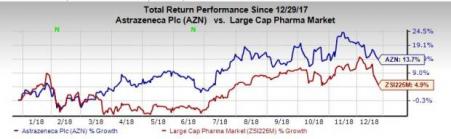
This year so far, AstraZeneca’s shares have risen 13.7% compared with the industry’s growth of 4.9%.
In a separate press release, AstraZeneca and Merck announced positive results from phase III SOLO-3 study of Lynparza, which was conducted as a post-approval commitment in agreement with the FDA. The results showed that BRCAm advanced ovarian cancer patients treated with Lynparza following two or more prior lines of chemotherapy experienced a statistically-significant and clinically-meaningful improvement in the primary endpoint of objective response rate (ORR) and the key secondary endpoint of progression-free survival (PFS) compared to chemotherapy. This is the fourth phase III study to report positive results for Lynparza. The companies plan to discuss the results with the FDA.
Astrazenca also announced that two phase III studies OLYMPUS and ROCKIES, evaluating pipeline candidate roxadustat for the treatment of patients with anaemia in chronic kidney disease (CKD), met their primary efficacy endpoint.
The OLYMPUS study was conducted on non-dialysis-dependent CKD patients, while the ROCKIES study was conducted on dialysis-dependent CKD patients.
While OLYMPUS demonstrated a statistically-significant and clinically-meaningful improvement in haemoglobin versus placebo, ROCKIES showed a similar improvement in haemoglobin versus epoetin alfa.
Roxadustat is a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), which is being jointly developed and commercialized by AstraZeneca and FibroGen.
Data from the phase III OLYMPUS and ROCKIES trials together with the efficacy and pooled safety data from the global phase III program conducted by AstraZeneca, FibroGen and Astellas will be part of the regulatory submission package in the United States and other major countries.
We remind investors that roxadustat was approved in China (its first regulatory approval) for the treatment of anaemia in chronic kidney disease patients on dialysis, earlier this week.
Zacks Rank
AstraZeneca currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Stocks from Zacks' Hottest Strategies
It's hard to believe, even for us at Zacks. But while the market gained +21.9% in 2017, our top stock-picking screens have returned +115.0%, +109.3%, +104.9%, +98.6%, and +67.1%.
And this outperformance has not just been a recent phenomenon. Over the years it has been remarkably consistent. From 2000 - 2017, the composite yearly average gain for these strategies has beaten the market more than 19X over. Maybe even more remarkable is the fact that we're willing to share their latest stocks with you without cost or obligation.
AstraZeneca PLC (AZN): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS): Free Stock Analysis Report
TESARO, Inc. (TSRO): Free Stock Analysis Report
Original post
Zacks Investment Research