We issued an updated report on Alnylam Pharmaceuticals Inc. (NASDAQ:ALNY) on Sep 18.
The company’s only approved drug Onpattro’s (patisiran) uptake has been strong since its launch. In August 2018, the FDA approved Onpattro lipid complex injection, a first-of-its-kind RNA interference (RNAi) therapeutic, for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The drug is the first and only FDA-approved treatment for this indication. It should drive revenues for the company as it will be an important treatment option for people suffering from this often fatal disease.
In September 2019, Alnylam announced the initiation of a study of Onpattro to expand the label of the drug. The company initiated a phase III APOLLO-B study on Onpattro for the treatment of ATTR amyloidosis with cardiomyopathy. The initiation of APOLLO-B represents a significant milestone for the company to explore the full potential of Onpattro for patients living with all types of ATTR amyloidosis.
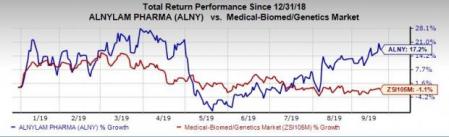
Shares of the company have gained 17.2% year to date against the industry’s decline of 1.1%.
The company is also evaluating several other candidates.
The company advanced givosiran, an investigational RNAi therapeutic in development for the treatment of acute hepatic porphyria (AHP). In June, Alnylam completed the rolling submission of a new drug application (NDA) to the FDA for the same. In August, the FDA accepted the company’s NDA for givosiran and granted a Priority Review for the same. The agency has set an action date of Feb 4, 2020, and indicated that it is not currently planning an advisory committee meeting as part of the NDA review. Givosiran is also under regulatory review in Europe.
Alnylam is also advancing lumasiran, an investigational RNAi therapeutic in development for the treatment of primary hyperoxaluria type I (PH1). It continues enrollment in ILLUMINATE-A, a global phase III pivotal study on lumasiran in children and adult PH1 patients with preserved renal function. The company expects to report top-line results from the study later this year, and if positive, will file for global regulatory approvals in early 2020.
The company is also evaluating inclisiran (formerly known as PCSK9si or ALN-PCSsc), an investigational RNAi therapeutic, in phase II ORION studies for hypercholesterolemia. The company’s partner, The Medicines Company (NASDAQ:MDCO) , reported new results for the drug in developing the treatment for hypercholesterolemia. The company intends to file an NDA later this year.
The successful development and subsequent approval of these candidates will be a huge boost for the company.
Alnylam has entered several collaboration deals for the development and commercialization of its broad pipeline of RNAi therapeutic candidates.
In April, the company and Regeneron Pharmaceuticals (NASDAQ:REGN) extended their collaboration agreement. Both companies will work together to discover, develop and commercialize new RNA interference (RNAi) therapeutics for a broad range of diseases by addressing disease targets expressed in the eye and the central nervous system (CNS), in addition to a select number of targets expressed in the liver. The companies plan to advance programs directed to 30 targets. Other candidates also might be introduced into clinical development during the initial five-year discovery period, which may extend.
With only one approved product in its portfolio, Alnylam derives a substantial amount of revenues from partnerships with companies like Sanofi (NASDAQ:SNY) , Takeda and Monsanto (NYSE:MON), among others. Therefore, the company is heavily dependent on its partnerships for its operations and pipeline development activities. Moreover, Alnylam is not the only company working on the development of RNAi-based therapeutics. Companies like Ionis, Sarepta Therapeutics and Roche Innovation Center are involved in the development of RNA-based drugs.
Alnylam currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth. Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Sanofi (SNY): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
The Medicines Company (MDCO): Free Stock Analysis Report
Original post