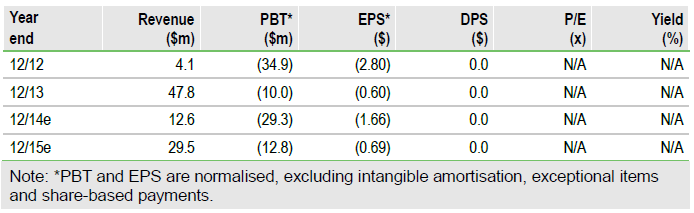
Teva launches Adasuve, Alexza raises $45m
Adasuve (Staccato loxapine) was launched in the US by Teva in early March, at a price of $145/dose (above our previous forecast of $80/dose). Alexza Pharmaceuticals Inc (ALXA.O) also raised $45m in debt financing, which we estimate could potentially extend its funding runway to the stage where Adasuve-related revenues would sustainably generate positive free cash flow, thereby lessening financing risks. We now estimate 2018 global sales of $337m (up from $213m, previously), and increase our valuation to $9.06/share (vs $6.05, previously), strengthening the investment case.
Adasuve US price well ahead of previous guidance
Adasuve’s US launch price of $145/dose was above the $75-125 range previously estimated by Alexza management. The product was also granted a ‘C’ billing code for reimbursement by Centers for Medicare & Medicaid Services (CMS), which may help ease product reimbursement in outpatient settings and support adoption.
$45m financing extends Alexza’s financial flexibility
On 18 March, Alexza issued $45m in senior secured notes (bearing 12.25% pa interest) and five-year warrants to purchase 345,561 common shares at $0.01/share. Alexza expects the net proceeds of $41m will provide sufficient funds to maintain operations into 2015. Alexza can also draw down another $10m from its Teva loan facility. Hence, we project that both these sources of funds could enable the firm to reach the point in time (estimated in H216) where Adasuve-related royalty and milestone revenue from its partners should exceed Alexza’s operating costs, assuming R&D investments do not increase materially.
EU roll-out continues
Since January 2014, EU partner Ferrer launched Adasuve in Spain (triggering a $1m milestone payment to Alexza) and in Romania. Adasuve was introduced in Germany and Austria in H213 and further EU launches are expected in 2014 and 2015.
Valuation: rNPV of $145.4m represents upside
We calculate an rNPV for Adasuve and AZ-002 at $145.4m. Adding $14.2m in estimated net cash (at Q413) gives a $9.06 per share valuation for the firm. This offers significant upside to the current share price. We also do not specifically value the Staccato platform technology, which we believe can be extended into other pharmaceutical ingredients and provide further value-creation opportunities.
To Read the Entire Report Please Click on the pdf File Below