Abbott Laboratories (NYSE:ABT) ABT recently confirmed that it has adequate capacity and supply to support the expanded use of HeartMate3 heart pump to meet the rising demand for mechanical circulatory support (MCS) devices for the effective treatment of advanced heart failure. This announcement came on the heels of Medtronic (NYSE:MDT)’s MDT last week’s decision to discontinue the global distribution and sale of Medtronic’s competitive product HeartWare ventricular assist device (HVAD).
Abbott’s HearMate 3 thus is expected to meet the excess demand that has been created due to the sudden discontinuation of Medtronic’s HVAD.
In addition, Abbott is working with physicians and health systems to ensure supply of left ventricular assist devices (LVADs) as well as training and education for physicians offering Abbott's HeartMate 3 to patients.
Abbott is expected to strengthen its electrophysiology and heart failure business and expand its customer base globally with adequate capacity and supply to meet the growing demand.
More on HeartMate 3
Abbott’s HeartMate 3 heart pump is a small, implantable mechanical circulatory support device for patients with advanced heart failure in need of either short or long-term support awaiting a heart transplant or who are not eligible for heart transplantation.
In a recent study published in the Annals of Thoracic Surgery regarding the risk of adjusted and propensity matched patients supported with the HeartMate 3 demonstrated that actuarial survival rates were 87% at one year and 84% at two years.
More in the News
Per Abbott’s management, life-savings results has been seen in patients treated with mechanical circulatory support devices and ensuring continued access to these devices is important for patients. Abbott is working to make sure that physicians have the support and training as they adopt HeartMate 3 to enhance outcomes for advanced heart failure patients.
Further, left ventricular assist devices (LVADs) remain a vital option for patients with advanced heart failure, either awaiting a heart transplant or not eligible for transplantation which can extend patients’ lives and restore quality of life. Abbott's HeartMate 3 heart pump is available to support physicians and patients globally in markets where the technology has been approved for use.
Recent Developments
In May 2021, Abbott announced a new trial focused on improving treatment for people simultaneously suffering from both atrial fibrillation (AFib) and heart failure. Notably, the trial will make use of Abbott’s technology, including cardiac ablation and sensors to monitor for both pulmonary artery pressure and abnormal heartbeats. The first-of-its-kind trial intends to offer new insights into more effective treatment for patients with AFib and heart failure -- a complex combination that has historically presented significant challenges to physicians.
In April 2021, Abbott announced the receipt of CE Mark for TriClip Transcatheter Tricuspid Valve Repair System -- the first-of-its-kind minimally invasive tricuspid heart valve repair device to treat tricuspid (TR) regurgitation available in Europe. Notably, the clip-based therapy called TriClip G4 is a non-surgical heart valve repair system designed particularly to treat TR, or a leaky tricuspid valve.
Industry Prospects
Per a report by MARKETSANDMARKETS, the global electrophysiology market is projected to reach $10.6 billion by 2025 from $6.8 billion in 2020, at a CAGR of 9.1%. Rising incidence of heart failure and cardiac arrest cases, attributed to lifestyle habits such as smoking and excessive alcohol consumption, are the major factors driving the market.
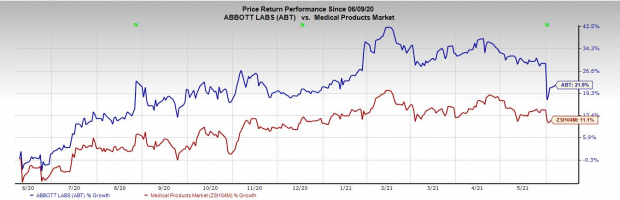
Price Performance
Shares of the company have gained 21.9% in a year’s time compared with the industry’s 11.1% rise.
Zacks Rank and Key Picks
Currently, the company carries a Zacks Rank #4 (Sell).
A few better-ranked stocks from the broader medical space are Envista Holdings (NYSE:NVST) Corporation NVST and IDEXX Laboratories, Inc. IDXX, each carrying a Zacks Rank #2 (Buy). You can see the complete list of Zacks #1 Rank (Strong Buy) stocks here.
Envista Holdings has an estimated long-term earnings growth rate of 26%.
IDEXX Laboratories has a projected long-term earnings growth rate of 20%.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 77 billion devices by 2025, creating a $1.3 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 4 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2022.
Click here for the 4 trades >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Medtronic PLC (MDT): Free Stock Analysis Report
Abbott Laboratories (ABT): Free Stock Analysis Report
IDEXX Laboratories, Inc. (IDXX): Free Stock Analysis Report
Envista Holdings Corporation (NVST): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
