Vitae Pharmaceuticals Overview
Vitae is a clinical stage biotechnology company focused on discovering and developing novel, small molecule drugs for diseases that represent large market opportunities where there are significant unmet medical needs.
Focusing in particular on type 2 diabetes and Alzeimer's. Boehringer Ingelheim GmbH is the collaborator.
Based in Fort Washington, PA, Vitae Pharmaceuticals (VTAE) scheduled a $60 million IPO on the Nasdaq with a market capitalization of $196 million at a price range mid-point of $12 for Wednesday, Sept. 24, 2014.
There are nine new IPOs scheduled for the week of September 22.
Management
Manager, Co-Managers: Stifel, BMO Capital Markets
Joint Managers: JMP Securities, Wedbush PacGrow
Valuation (Glossary)
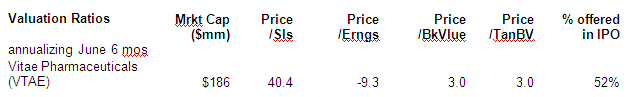
Conclusion
The rating is neutral plus. Company is currently in Phase 2 trials for type 2 diabetes. In Phase 1 trials for Alzheimer's.
Stockholders affiliated with Prospect Venture Partners, New Enterprise Associates, Venrock Associates and Atlas Ventures, indicated an interest in purchasing an aggregate of up to $11 million of IPO stock.
To put the conclusions and observations in context, the following is reorganized, edited and summarized from the full S-1 referenced above.
Business
VTAE is a clinical stage biotechnology company focused on discovering and developing novel, small molecule drugs for diseases that represent large market opportunities where there are significant unmet medical needs.
VTAE is developing a robust and growing portfolio of novel product candidates generated by Contour®, its proprietary structure-based drug discovery platform.
VTAE’s team of accomplished scientists utilizes Contour to rapidly discover highly potent and selective product candidates for validated but difficult-to-drug targets in multiple disease areas.
Clinical trials
VTAE’s most advanced product candidates include VTP-34072, which commenced the first Phase 2 clinical trial for the treatment of type 2 diabetes in July 2014, with data expected in the first half of 2015, and VTP-37948, which is in Phase 1 clinical trials for the treatment of Alzheimer's disease, or Alzheimer's, with data expected in the second half of 2014.
Collaborations
Both products are being developed by Boehringer Ingelheim GmbH, or BI, under separate collaborations. Due to the fact that the trials for VTP-34072 and VTP-37948 are being conducted by BI in Germany, no IND (investigational new drug) applications have been submitted in the United States to date.
These collaborations have provided VTAE with an aggregate of $152 million in funding as of June 30, 2014, including upfront license fees, research funding and success-based milestone payments as well as equity investments.
Preclinical studies
In addition, VTAE has several wholly-owned products advancing in preclinical studies, including VTP-43742 for the treatment of autoimmune disorders, with Phase 1 proof-of-concept expected by the end of 2015, VTP-38443 for the treatment of acute coronary syndrome, and VTP-38543 for the treatment of atopic dermatitis.
VTAE intends to advance and retain rights to these and other programs and product candidates that VTAE believes can be developed and commercialized by us, and to strategically partner where doing so can accelerate a program and generate non-dilutive capital for us.
Structured-based drug discovery
VTAE is a structure-based drug discovery company, and has leveraged its expertise to create a growing portfolio of novel, potent and selective product candidates.
VTAE utilizes Contour to discover and develop product candidates for validated therapeutic targets against which the industry has traditionally struggled to develop drugs due to challenges related to potency, selectivity, pharmacokinetics, or patentability issues.
VTAE refers to these targets as "difficult-to-drug." Contour's computational software uses artificial intelligence and sophisticated algorithms to model the assembly of molecular fragments, which are chemical structures consisting of one to several atoms, into fully elaborated, drug-like structures that precisely fit each target's 3-dimensional binding site. These molecules are then assessed by Contour's state-of-the-art scoring function to identify the most promising and drug-like structures.
Together, these functions allow VTAE to rapidly focus on those structures with the highest potential from among hundreds of billions of possibilities for a given biologic target. VTAE chemically synthesizes, comprehensively tests and critically evaluates these novel structures rapidly, iterating each new data set back into the design process until VTAE identifies product candidates with demonstrable first- or best-in-class potential.
VTAE’s experienced scientists are experts in the related disciplines of structural biology, molecular modeling (i.e., the design of drugs using computers), medicinal chemistry and biology. VTAE’s scientists utilize its platform and approach for each of its product candidates to rapidly overcome discovery obstacles.
VTAE has achieved animal proof-of-concept with a qualified product candidate in less than 18 months from the initiation of a program.
Dividend Policy
No dividends are planned.
Intellectual Property
Each of VTAE’s most advanced product candidates is the subject of patents and patent applications for composition of matter and methods of treatment in major markets worldwide.
These patents and patent applications, if granted, are expected to provide VTAE with intellectual property protection for all of VTAE’s current product candidates until 2030 and beyond.
VTAE intends to continue to expand its intellectual property protections by seeking and maintaining domestic and international patents on inventions that are commercially important to its business. VTAE will also relies on know-how and continuing technological innovation to develop and maintain its proprietary position.
Competition
If VTAE’s product candidates are approved, they will compete with currently marketed drugs and potentially with product candidates currently in development focusing on the same mechanism of action which include:
•11b HSD1: VTAE believes that Bristol-Myers Squibb Company (NYSE:BMY) or BMS, Eli Lilly and Company (NYSE:LLY), and Roche Holding (OTC:RHHVF) are studying their 11b HSD1 inhibitors in clinical trials.
•BACE: VTAE believes that Merck & Company Inc (NYSE:MRK), Astrazeneca Plc (NYSE:AZN) and Eisai Co., Ltd. (TOKYO:4523) in collaboration with Biogen Idec Inc (NASDAQ:BIIB) are studying BACE inhibitors in clinical trials.
•RORgt: VTAE believes that a number of companies including large pharmaceutical companies and large biotech companies are actively assessing RORgt inhibitors in preclinical studies.
•LXRb: VTAE believes that BMS is studying an LXRb inhibitor in cardiovascular clinical trials and Alexar Therapeutics, Inc. is developing an LXRb inhibitor for dermatologic conditions.
5% Stockholders
- Prospect Venture Partners 15.2 %
- New Enterprise Associates 17.8 %
- Venrock Associates 15.6 %
- Atlas Venture 11.2 %
- Boehringer Ingelheim R&D Beteiligungs GmbH 10.8 %
- Allergan, Inc. 5.6 %
- Peter Barrett, Ph.D. 11.2 %
- Bryan Roberts, Ph.D. 15.6%
Use of Proceeds
VTAE intends to use the $53 million in proceeds from its IPO as follows along with cash equivalents:
$14.0 million to fund the costs for progressing its RORgt program through Phase 1 clinical development, which consists of two trials, a single dose trial and a two week multiple dose trial, both for VTP-43742 and to identify backup compounds;
$4.0 million to fund the costs for progressing VTP-38443 through filing an IND in preparation for a Phase 1 clinical trial for the treatment of ACS;
$4.0 million to fund the costs for progressing VTP-38543 through a single dose Phase 1 clinical trial for the treatment of atopic dermatitis;
$20.0 million to fund its continued discovery efforts to identify additional drug candidates for new therapeutic molecular targets, including its immuno-oncology efforts;
$9.0 million for debt maintenance (its credit facility bears interest at 8.85% per annum and the final payment is due in October 2015); and
the remainder for working capital and other general corporate purposes.
The full IPO calendar is available at IPOpremium.
Disclaimer: This VTAE IPO report is based on a reading and analysis of VTAE’s S-1 filing, which can be found here, and a separate, independent analysis by IPOdesktop.com. There are no unattributed direct quotes in this article.
