Detailed data on rolapitant presented
TESARO Inc (NASDAQ:TSRO) presented detailed data of three Phase III trials of rolapitant (one for patients receiving moderately emetogenic chemotherapy [MEC] and two for highly emetogenic chemotherapy [HEC]), which reinforced the differentiated attributes of the product compared to other products in the same class. The company also announced two planned Phase III trials of Niraparib, bringing the total Phase III programmes to four for this drug.
Rolapitant trials detailed
TESARO presented detailed Phase III data of three trials (HEC1, HEC2 and MEC) at the 2014 ASCO (American Society of Clinical Oncology) annual meeting. These detailed data (top-line results were reported previously in press releases) continue to build a differentiated product profile for the drug: superb safety, long half-life (which translates into good efficacy) and multiple forms of administration (oral and IV).
New Phase III trials planned for niraparib
TESARO announced two new Phase III trials of niraparib as a maintenance therapy, one in first-line ovarian cancer and another in first-line small cell lung cancer (SCLC). These two programmes bring the total Phase III programmes to four and could potentially make the drug eligible to 30,360-35,240 patients, significantly larger than the size of target patients covered by the previous two Phase III trials, platinum sensitive, relapsed high-grade serous ovarian cancer and germline BRCA mutation positive, advanced or metastatic breast cancer.
Immunotherapy pipeline in progress
TESARO’s Immuno-oncology pipeline consists of five antibodies against various immune checkpoints at pre-clinical stages of development. Investigational new drug (IND)-enabling work has started for the lead candidate, anti-PD1 antibody TSR-042, which should enter clinical trials in mid-2015.
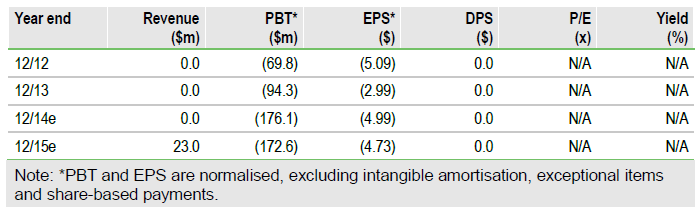
Valuation: Still attractive with more upside in 2015
We have slightly lowered our valuation of TESARO to $1,806m, or $50.3/share from previously $1,831m, or $51.0/share, due to higher R&D cost estimates in 2014. Our valuation does not include any value of the early-stage immune checkpoint portfolio as the candidates are still in preclinical development or the two new niraparib indications as the trial accruals have not started yet.
To Read the Entire Report Please Click on the pdf File Below