Printemps à Paris
Neovacs (PARIS:ALNEV) has refocused onto IFNα-Kinoid in lupus (SLE). The planning to start a Phase IIb lupus study in mid-2015 is now well advanced: a small US lupus study could start in H116. To retain a greater share of the profits, if approved, Neovacs then plans to run a Phase III trial. The company has stated that a partner may fund a second Phase III. Year-end cash was €5.6m. Further equity funding of about €16.1m is potentially available. The pre-dilution indicative value is €39.9m based on lupus.
Phase IIb trial in Systemic lupus erythematosus (SLE)
Neovacs is setting the basis for the SLE strategy. The scientific advisory board has been realigned and has approved the development plan; a US subsidiary has been established to access the US market; an expanded supply agreement was entered into with Stellar Biotechnologies for Keyhole limpet hemocyanin, a primary Kinoid component. Neovacs plans to run a 160-patient European, Asian and Latin American Phase IIb in SLE. This will use a primary endpoint of neutralisation of the IFNα-signature with a secondary endpoint of a detailed composite clinical efficacy measure. This trial is planned to start in mid-2015 and could report by late 2016. A US Phase IIa with 50 patients could start in early 2016, possibly reporting by Q317, formerly late 2016. The strategy is to fund at least one Phase III with a partner sought to fund the second Phase III. Neovacs aims to sell direct in the EU.
Financials: Cash of €5.6m with significant equity line
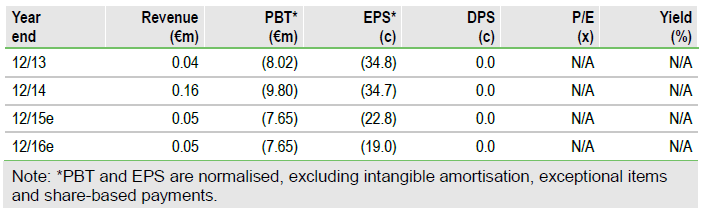
The FY14 results showed an operating loss of €9.65m of which €7.76m was R&D. Neovacs gained a tax credit of €2.3m; we assume that similar tax credits are received in 2015 and 2016 aiding cash flow. Neovacs had year-end cash of €5.6m after equity issued of €9.7m. Neovacs has about a €16.1m remaining contingent equity line with Kepler Chevreux after issuing 400,000 shares in March 2015. A further c €3.1m of this tranche can be drawn by November. The forecast assumes that €6.5m is drawn down in 2016 with a further €6.5m then available.
Valuation: Risk adjusted pre-dilution NPV of €39.3m
Edison’s risk-adjusted, pre-dilution, post-tax indicative value remains at €39.9m pending an update on the clinical programme and partnering strategy. It now rests entirely on IFNα-Kinoid for SLE. The probability of SLE success remains at 20%. The value scenario runs to 2033 to incorporate US biological exclusivity. Management assumes there will be a profit share with a US partner and envisages direct sales in the commercially smaller EU market.
To Read the Entire Report Please Click on the pdf File Below