Sharpening strategy
Nanobiotix, (NANOB) is refining and shaping its strategy to realise value from NBTXR3, a potential treatment to improve the benefits of radiotherapy. Data from the ongoing soft tissue sarcoma trial suggest a positive profile that could allow for earlier than anticipated approval in Europe in 2016. A pivotal trial is now being planned and could start by year end 2014. Our valuation has more than doubled to €282m or €26.2 per share reflecting the updated strategy and higher pricing that NBTXR3 could attract.
Positive STS data could lead to earlier sales
Following additional positive data in soft tissue sarcoma (STS), Nanobiotix now intends to conduct a pivotal STS trial, which is planned to start by year end 2014. This could allow for first approval (CE mark as a medical device) in 2016, around a year earlier than we anticipated. Although Nanobiotix is unlikely to seek reimbursement in this first indication, this could allow for first self-pay sales in 2016 and help raise awareness of NBTXR3.
Potential benefits could lead to higher reimbursement
Following feedback from payers, if NBTXR3 can improve survival in patients, it is likely to be viewed favourably. Hence, Nanobiotix will seek reimbursement once such data are available. Larger clinical trials had always been included in the development plan to gain acceptance in the medical community. However, we have now adjusted our NBTXR3 pricing assumptions to reflect the refined strategy aiming for “gold standard” overall survival data in liver cancer.
Increasing peak sales to €1.4bn
We have increased our NBTXR3 price from €12,000 to €20,000 per patient, which together with the inclusion of STS raises our peak sales forecast to €1.4bn (from €700m). We only include NBTXR3 in the planned indications of head and neck, oesophageal, rectal, STS and liver cancers in Europe, the US and Asia. This is a patient population of around 270,000, versus around six million new cases of cancer in developed world (2012), of which around 50-60% receive radiotherapy.
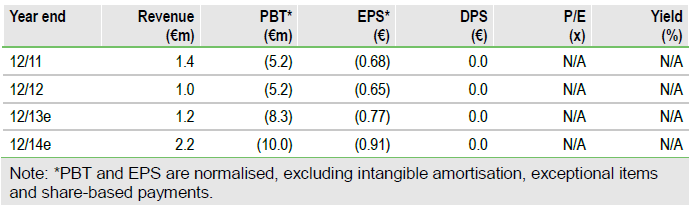
Valuation: Risk-adjusted NPV of €282m
Our valuation is now €282m or €26.2/share based on our increased peak sales, with no changes to our risk adjustments. Estimated net cash of €3.6m at end December 2013 should be sufficient to fund operations to mid-2014, but not to execute on the updated strategy, in particular the start of a pivotal STS trial.
To Read the Entire Report Please Click on the pdf File Below