U(CB)-turn on tozadenant
UCB has returned its global rights to tozadenant to Biotie Therapies Oyj (BTH1V.HEL). This follows a portfolio review by UCB and does not reflect any concerns over the drug’s efficacy or safety. While disappointing to lose the expertise and funding from UCB, we remain hopeful that Biotie can secure a new partner for the A2a antagonist that produced the most compelling Phase IIb data in its class for Parkinson’s disease. UCB is committed to making tozadenant a Phase III-ready asset, although the terms of the transfer of rights are to be determined. We therefore maintain our valuation at €235m (€0.52/share).
Tozadenant fundamentals unchanged
In December 2012 Biotie reported highly encouraging results from a 420-patient Phase IIb study of tozadenant, in mild-to-moderate Parkinson’s disease. Significant reductions in ‘off’ time, increases in ‘on’ time, reductions in UPDRS scores, improvements in clinician/patient assessments and acceptable safety data indicate a more robust profile than other A2a antagonists (preladenant and istradefylline).
Disappointing given UCB’s prior commitment
These results prompted UCB to pay Biotie $20m (€15m) in February 2013 to exercise its option to advance tozadenant, effectively funding the required pre-clinical and manufacturing work, with Phase III studies planned to start in H115. UCB was to fund all clinical/regulatory work (low $100ms for six years), on top of its original 2010 deal to pay up to $340m in milestones and double-digit royalties.
Seeking a smooth transition
The decision to drop tozadenant is the result of UCB’s portfolio review, although the failure in 2013 of Merck & Co’s preladenant in Phase III studies may have had an impact. However, we view the investment case in tozadenant as compelling (given the Phase IIb data). As a Phase III-ready product, tozadenant will arguably be a more valuable asset than 12 months ago. An end-of-Phase II meeting with the FDA in Q214, which could consider the Phase IIb trial as one of two pivotal studies required for approval, is now an important share price and partnering catalyst.
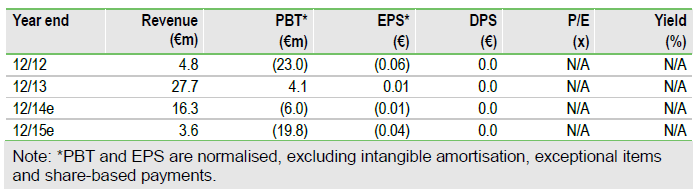
Valuation: Maintained at €235m, €0.52 per share
We understand investor disappointment (shares -15% to €0.23) at UCB’s decision, but reserve judgement on its impact on our valuation – the extra risk of requiring a new partner may be offset by the work to make tozadenant a more attractive asset. We maintain our valuation until the terms of the UCB transfer and the result of the FDA meeting are known. Q114 results on 9 May should provide clarity.
To Read the Entire Report Please Click on the pdf File Below