A maturing strategy
The completion of Biotie Therapies’s, (F8S.BE) portfolio review sets out a new strategic path as the company aims to transition from a search/develop/license model to a more integrated approach, particularly in bringing products to the market. The option to acquire Neurelis for NRL-1 (intranasal diazepam for epilepsy), a product that could be commercialised by Biotie in the US, is a prime example, and we anticipate further deals for assets with similar attributes. Securing a partner for SYN120 and/or co-funding for BTT-1023 in a Phase II study for a rare autoimmune liver disorder are other potential near-term catalysts.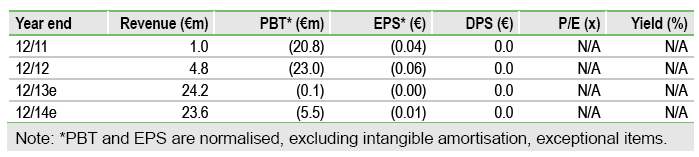
An integrated path
Having successfully developed and partnered Selincro (alcohol dependence; marketed in EU) with Lundbeck and tozadenant (Parkinson’s disease; Phase III to start H115) with UCB, Biotie has a strong financial position (€43m at Q313e) from which to seek new pipeline opportunities. The Neurelis deal (An option in epilepsy) provides a product with a relatively fast-track to approval in an established market requiring a small, specialist sales force which Biotie could establish. Biotie is conducting the manufacturing and pre-clinical toxicology work and expects to exercise its option to acquire Neurelis (for $8.75m in new shares) in H114. Pivotal PK studies should start by mid-2014, take two years to complete, suggesting potential approval (using the 505(b)(2) equivalence pathway) and launch in 2017.
Near-term developments
The review also highlighted some important near-term developments. Biotie reiterated its desire to secure a partner for SYN120, a 5HT6/2a antagonist with potential for Alzheimer’s disease and other cognitive disorders, with discussions at an ‘advanced stage’. We expect a deal within the next six months, although likely to be back-end loaded with a relatively modest upfront fee. For BTT-1023 (VAP-1 antibody) a development path forward in primary sclerosing cholangitis (PSC) has been identified, a niche but potentially lucrative market opportunity. Co-funding is expected to be secured for a Phase II proof-of-concept study, providing Biotie with the prospect of minimal investment (low single €ms over two-three years) to generate a more compelling partnering package for the product.
Valuation: Adjusted for cash to €229m
We maintain our rNPV of Biotie’s key products – Selincro, tozadenant and SYN120 – at €186m, but adjusting for estimated cash at end-Q313 of €43m (vs €45m at Q213), the overall valuation is now €229m (vs €231m), or €0.51 per share. Upside potential therefore exists from the exercising of the option on NRL-1 and co-funding to advance BTT-1023 for PSC, products not currently in our valuation model. Securing a partner for SYN120, and/or licensing activity to bring in new and complementary pipeline assets, would boost sentiment further.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Biotie Therapies: An Integrated Path
Published 10/14/2013, 08:13 AM
Updated 07/09/2023, 06:31 AM
Biotie Therapies: An Integrated Path
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.