The one into two at 10
January 2013 brought a clear safety and dose signal in the BI-505 Phase I for relapsed and refractory multiple myeloma (MM). In preclinical studies, BI-505 showed a tumour cell killing effect possibly by making myeloma cells susceptible to apoptosis. The Phase I was dose escalating with a safety end point. Interestingly but anecdotally, seven of 29 patients (24%) on extended dosing showed stable disease. BI-505 will progress to a small Phase IIa during 2013. BioInvent (BINV.ST) intends to partner BI-505
The one: BI-505 is crucial to current value
BI-505 is the remaining clinical programme from the January 2012 portfolio. Hence, gaining maximum value is important for investors. A deal by GenMab in August 2012 with J&J on a Phase I MM antibody, daratumumab, shows possible value. GenMab, a larger company, gained $55m upfront and $88m in equity with milestones and a 10%+ royalty. However, such Phase I deals are infrequent and BioInvent investors need to be prepared to fund BI-505 to add value by generating Phase II efficacy data.
Into two at 10: BI-505 progression to Phase II
BI-505 blocks the function of the cell surface adhesion molecule ICAM-1 (CD54). In the cancer literature, myeloid cells show cell-adhesion mediated drug resistance if attached to other cells. In BioInvent’s patent EP 2468775 (priority December 2006), ICAM blocking is claimed to cause apoptosis (cell suicide). The 35-patient dose escalation study in relapsed and refractory MM tested 11 dose stages of 0.0004mg/kg to 20mg/kg. BI-505 at 10mg/kg saturated the ICAM sites on bone marrow myeloma cells; this dose will be used for Phase II. Stable disease (based on M protein biomarker levels) was seen in 24% of the 29 patients who received extended dosing over 0.06mg/kg (level 6). The likelihood of success remains at 30%.
Valuation: Cash conservation to rebuild the portfolio
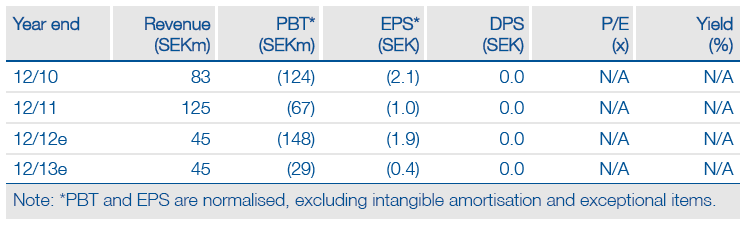
BioInvent had Q3 ytd cash of SEK153m with revenues of SEK 34m. Estimating year-end 2012 cash is difficult due to the actual H212 restructuring costs; SEK25-40m seems a probable range. The company needs to invest in BI-505, bring forward two other candidates into Phase I and support n-CoDeR. Operating cash costs in 2013 should be SEK75m. The small Phase IIa BI-505 study due to start sometime in 2013 will be inexpensive; a broader Phase IIb may eventually follow. Collaborations may generate milestones in 2013 if partners designate clinical candidates. This could give funding into 2014 before any BI-505 deal value, if partnered in 2013.
To Read the Entire Report Please Click on the pdf File Below.
January 2013 brought a clear safety and dose signal in the BI-505 Phase I for relapsed and refractory multiple myeloma (MM). In preclinical studies, BI-505 showed a tumour cell killing effect possibly by making myeloma cells susceptible to apoptosis. The Phase I was dose escalating with a safety end point. Interestingly but anecdotally, seven of 29 patients (24%) on extended dosing showed stable disease. BI-505 will progress to a small Phase IIa during 2013. BioInvent (BINV.ST) intends to partner BI-505

The one: BI-505 is crucial to current value
BI-505 is the remaining clinical programme from the January 2012 portfolio. Hence, gaining maximum value is important for investors. A deal by GenMab in August 2012 with J&J on a Phase I MM antibody, daratumumab, shows possible value. GenMab, a larger company, gained $55m upfront and $88m in equity with milestones and a 10%+ royalty. However, such Phase I deals are infrequent and BioInvent investors need to be prepared to fund BI-505 to add value by generating Phase II efficacy data.
Into two at 10: BI-505 progression to Phase II
BI-505 blocks the function of the cell surface adhesion molecule ICAM-1 (CD54). In the cancer literature, myeloid cells show cell-adhesion mediated drug resistance if attached to other cells. In BioInvent’s patent EP 2468775 (priority December 2006), ICAM blocking is claimed to cause apoptosis (cell suicide). The 35-patient dose escalation study in relapsed and refractory MM tested 11 dose stages of 0.0004mg/kg to 20mg/kg. BI-505 at 10mg/kg saturated the ICAM sites on bone marrow myeloma cells; this dose will be used for Phase II. Stable disease (based on M protein biomarker levels) was seen in 24% of the 29 patients who received extended dosing over 0.06mg/kg (level 6). The likelihood of success remains at 30%.
Valuation: Cash conservation to rebuild the portfolio
BioInvent had Q3 ytd cash of SEK153m with revenues of SEK 34m. Estimating year-end 2012 cash is difficult due to the actual H212 restructuring costs; SEK25-40m seems a probable range. The company needs to invest in BI-505, bring forward two other candidates into Phase I and support n-CoDeR. Operating cash costs in 2013 should be SEK75m. The small Phase IIa BI-505 study due to start sometime in 2013 will be inexpensive; a broader Phase IIb may eventually follow. Collaborations may generate milestones in 2013 if partners designate clinical candidates. This could give funding into 2014 before any BI-505 deal value, if partnered in 2013.
To Read the Entire Report Please Click on the pdf File Below.